How does Folliculin Regulate mTORC1 Activity?
6 Sep 2023
Our cells need to be able to sense and respond to our body’s needs. Mechanistic target of rapamycin complex 1 (mTORC1) is a protein in our cells that acts as a central hub for this sensing ability. It can sense several inputs and can turn on or off different pathways (branches) in response to these stimuli. It is often described as a single on/off switch however this process is likely to be far more complicated than that. A recent paper by Li et al., has described specific regulation of different mTORC1 branches.
Why is this relevant to Birt-Hogg-Dubé Syndrome (BHD)? BHD is caused by mutations in the gene folliculin (FLCN). The authors of the study found that FLCN was capable of specifically modifying mTORC1 function. It was able to turn on a specific branch of mTORC1 signalling without effecting the other pathways.
It has been shown previously that one of these mTORC1 branches is important for kidney tumour development in BHD. This pathway controls the activation of 2 related proteins called TFE3 and TFEB. These proteins are known as transcription factors. When they are turned on, they move from the cytoplasm to the nucleus (where DNA is located) and can trigger the expression of other proteins in our cells. Normally, when mTORC1 is active it modifies TFE3 and TFEB, so they stay in the cytoplasm (see the diagram below). This process is dependent on FLCN. So when cells are lacking FLCN, mTORC1 can no longer keep TFE3 and TFEB in the cytoplasm and they move to the nucleus. This results in expression of proteins that wouldn’t happen in cells containing FLCN and can drive tumour growth.
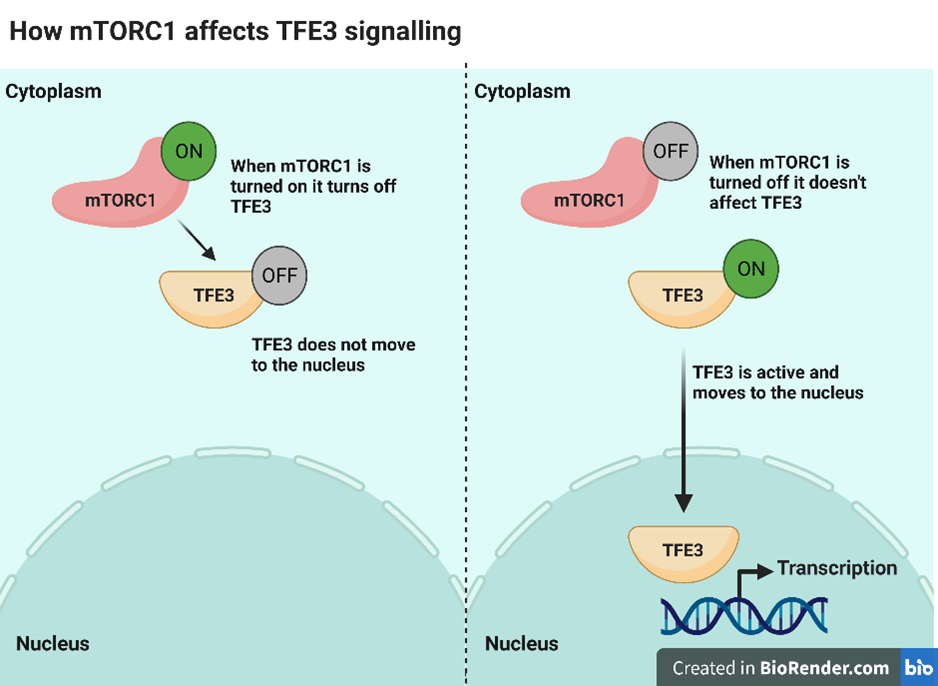
The authors of this present study have built upon this knowledge to understand how FLCN is able to influence TFE3 signalling through mTORC1 without modifying other branches. They found that FLCN activates another protein called RagC. RagC physically recruits TFE3 to an area of the cell where it can be turned off by mTORC1. TFE3 is kept in the cytoplasm and unable to move to the nucleus. The researchers made a special form of RagC that was always on and didn’t need FLCN to activate it. They saw that this active form of RagC could turn off TFE3 signalling in cells that were lacking FLCN. This meant that this whole process is dependent on the activity of FLCN. This process could also happen without affecting any of the other functions of mTORC1. Additionally, when TFE3 is turned on it can drive the expression of other proteins that further activate mTORC1. This is known as a positive feedback loop and results in the hyperactivation of mTORC1.
Previous work has shown conflicting roles for mTORC1 in BHD. In some models with reduced FLCN, mTORC1 activation was shown to be reduced. However, in samples derived from BHD kidney tumours, hyperactivation of mTORC1 was observed. The mechanism proposed in this study has helped address this conflicting evidence. Activity of mTORC1 is often measured by looking at proteins that are turned on or off by mTORC1. The variations in these models could be explained by different researchers looking at proteins in different branches of mTORC1 signalling. For example, if one researcher looked at TFE3 activity they may have concluded that mTORC1 activation was reduced. However, if another group looked at a protein in another branch of mTORC1 signalling, they may have found hyperactivity of mTORC1.
This work is important because it helps us better understand how loss of FLCN can potentially lead to cancer development. Knowing which proteins FLCN interacts with, and the effect of that interaction can help guide the development of new anti-cancer treatments.
